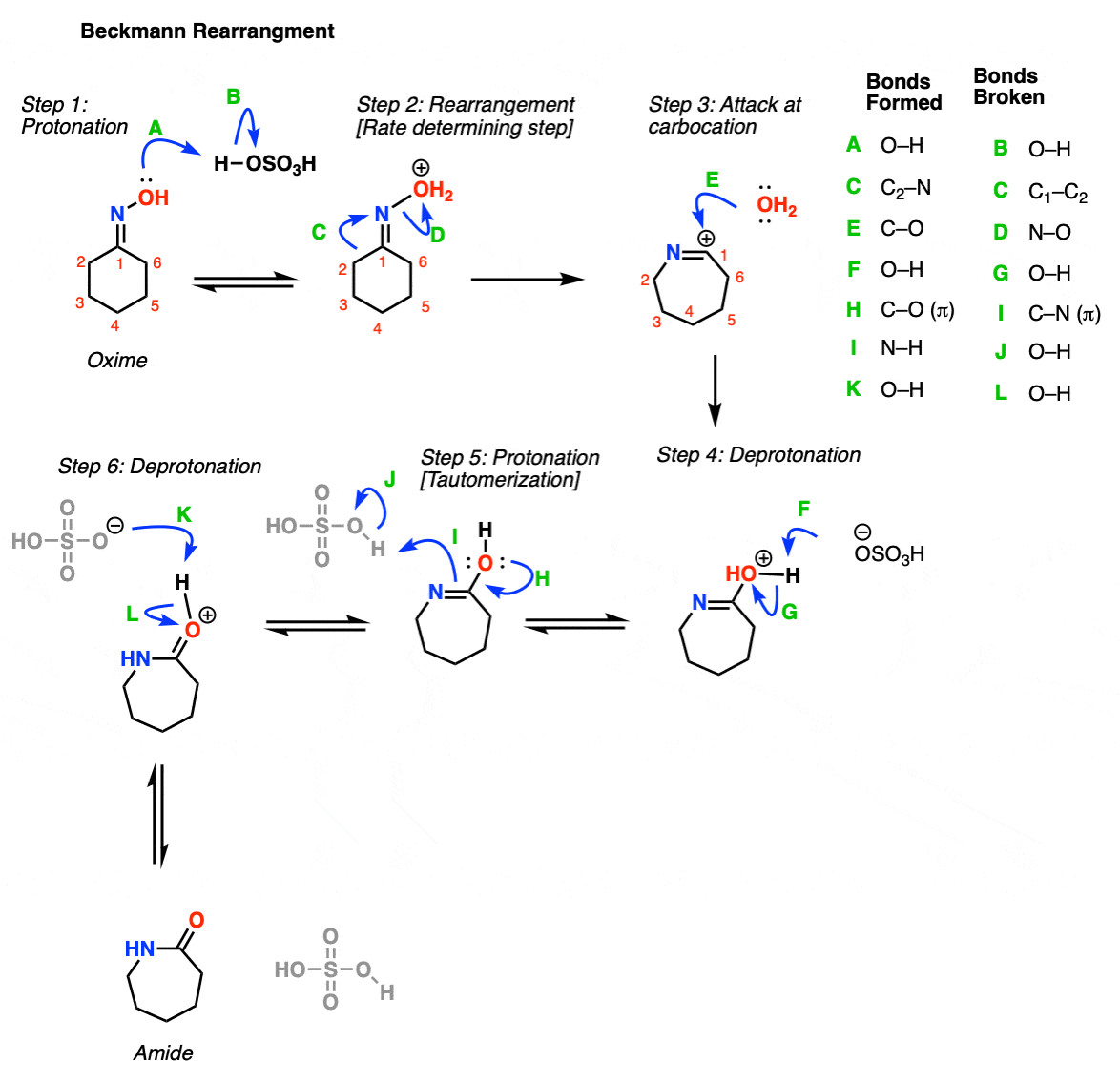
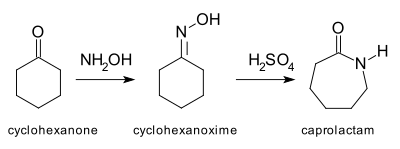
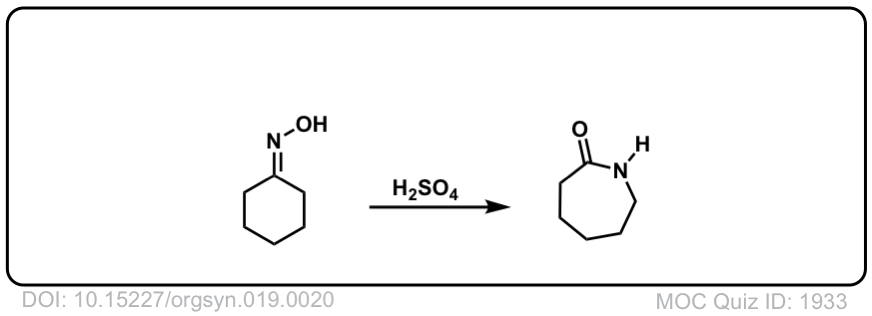
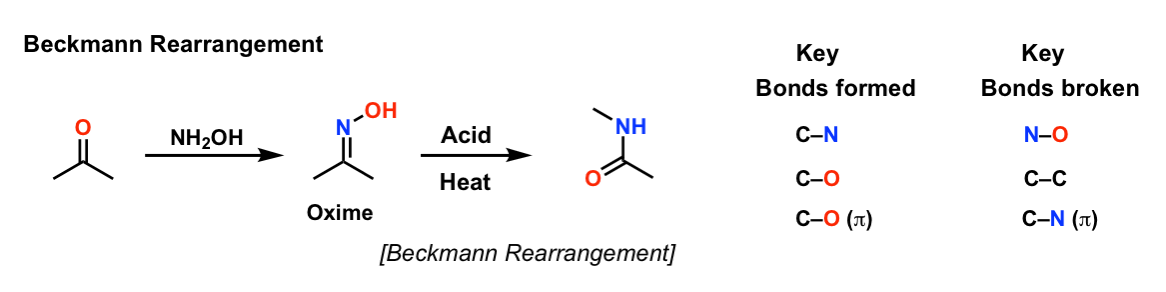
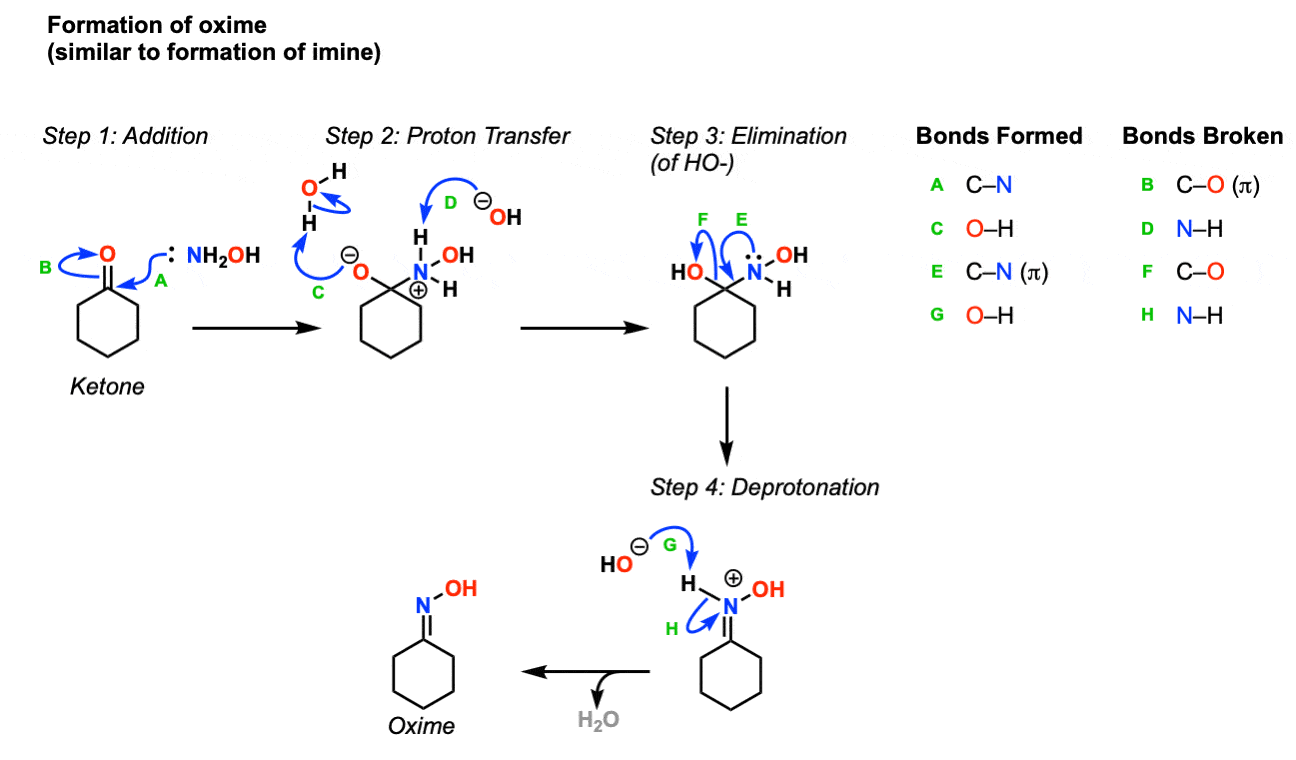
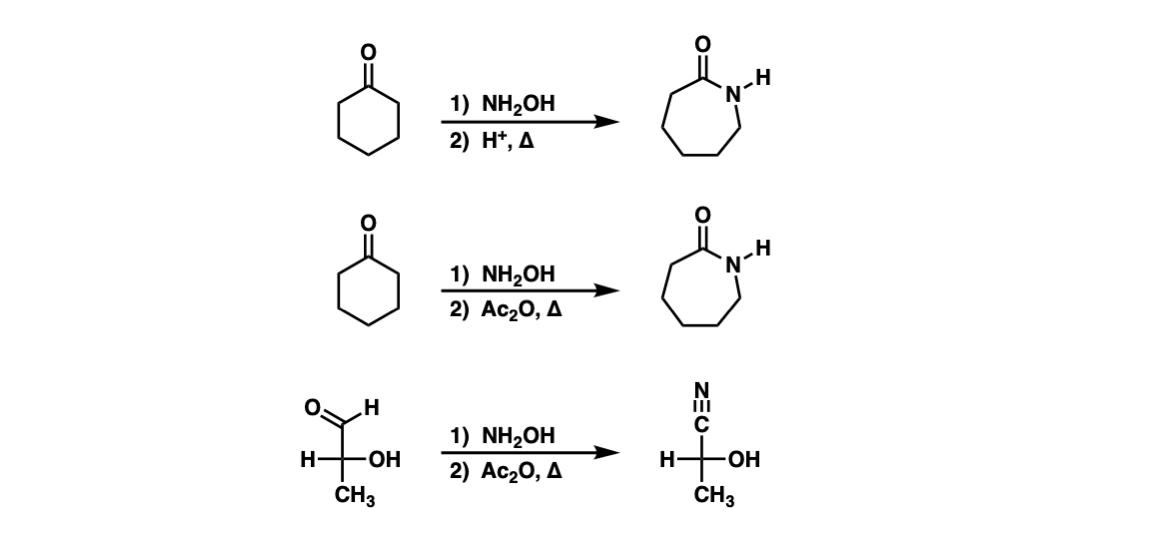
Cu(OTf)2-catalyzed Beckmann Rearrangement of Ketones Using Hydroxylamine-O-sulfonic Acid (HOSA).,Synthesis - X-MOL

Zinc(II)-Catalyzed Synthesis of Secondary Amides from Ketones via Beckmann Rearrangement Using Hydroxylamine-O-sulfonic Acid in Aqueous Media

Dichloroimidazolidinedione-Activated Beckmann Rearrangement of Ketoximes for Accessing Amides and Lactams | The Journal of Organic Chemistry

Cu(OTf)2-catalyzed Beckmann Rearrangement of Ketones Using Hydroxylamine-O-sulfonic Acid (HOSA). - Abstract - Europe PMC

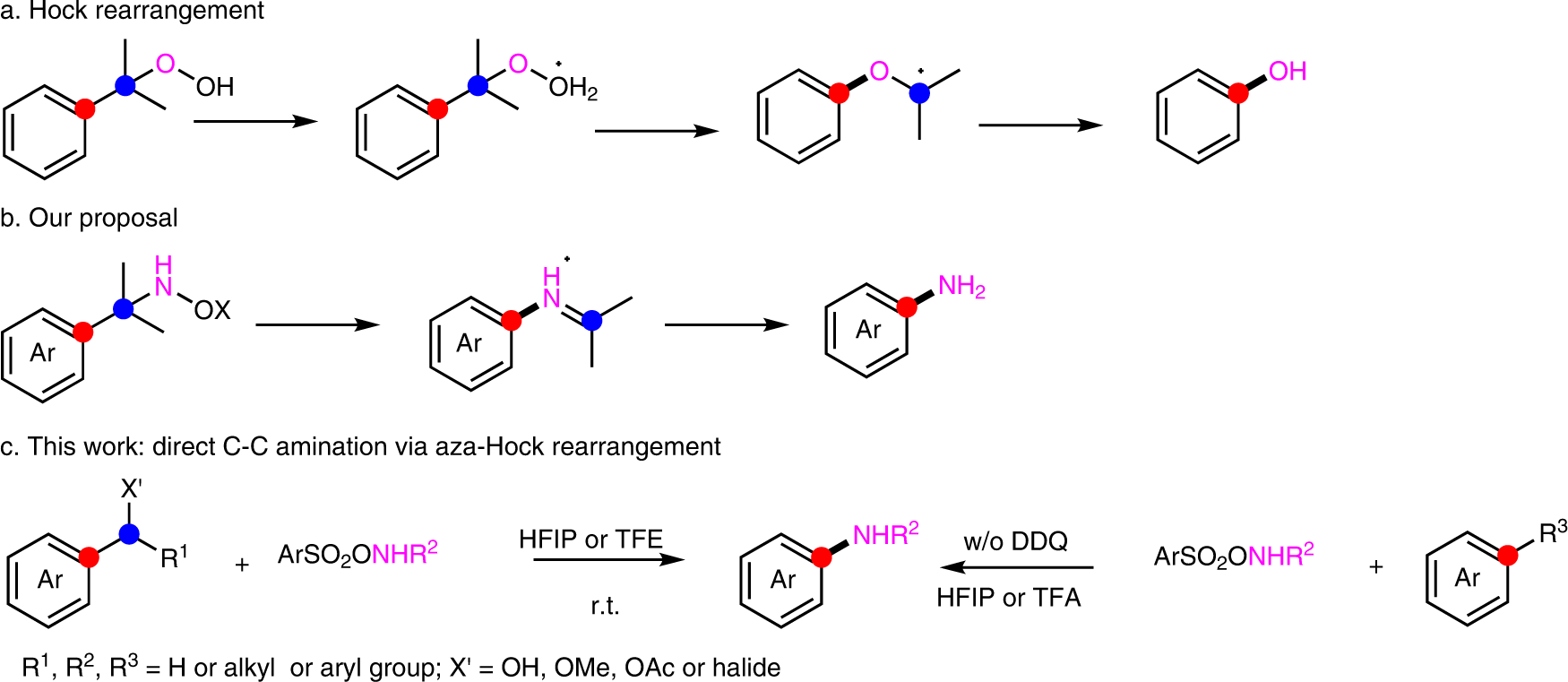
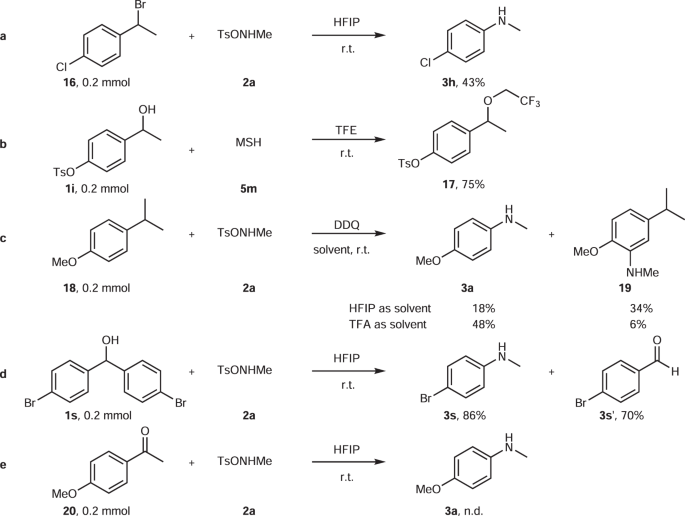
Scope and Mechanism of a True Organocatalytic Beckmann Rearrangement with a Boronic Acid/Perfluoropinacol System under Ambient Conditions | Journal of the American Chemical Society
![PDF] Cu(OTf)2-Catalyzed Beckmann Rearrangement of Ketones Using Hydroxylamine-O-sulfonic Acid (HOSA) | Semantic Scholar PDF] Cu(OTf)2-Catalyzed Beckmann Rearrangement of Ketones Using Hydroxylamine-O-sulfonic Acid (HOSA) | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0615b0928d07b0fb565394b813ca3929843b6cea/1-Figure1-1.png)
PDF] Cu(OTf)2-Catalyzed Beckmann Rearrangement of Ketones Using Hydroxylamine-O-sulfonic Acid (HOSA) | Semantic Scholar